A verbatim copy of
COMPUTATION AND THE SINGLE NEURON
by Christof Koch
taken from NATURE, 16 January, 1997
annotated and with two appendicesa1
a2
by M.Robert Showalter
Evidence and argument are presented here supporting the position that
current neuroscience data cited by Koch and connected to Koch's article,
combined with the zoom FFT EEG measurements of David Regan, strongly
supports the Showalter-Kline (S-K) passive conduction theory(1).
The S-K theory appears to permit memory, and to imply processing speeds
and logical capacities that are much more brain-like than permitted by
the current Kelvin-Rall conduction model. The annotation of Professor Koch's
article here is believed to be a good way to show how S-K theory fits in
with what is now known and thought about neural computation. I argue
that Regan's data, combined with the data cited and interpreted by Koch,
requires the sort of sharp resonance-like brain behavior that occurs under
S-K, that cannot occur under Kelvin-Rall.
Neurons and their networks underlie our perceptions, actions,
and memories. The latest work on information processing and storage at
the single-cell level reveals previously unimagined complexity and dynamism.
Over the past few decades, neural networks have provided the dominant
framework for understanding how the brain implements the computations necessary
for its survival. At the heart of these networks are simplified and static
models of nerve cells. But neuroscience is undergoing a revolutiona0,
and one consequence is that the picture of how neurons go about their business
has altered.
a0. This annotation of Koch's article presents a new idea adapted
to that revolution. The idea is that the current passive neural transmission
equation (Kelvin-Rall) should be replaced with a new equation (Showalter-Kline,
or S-K) that includes cross effects that yield two conduction modes, one
much like that of Kelvin-Rall, the other a lower dissipation mode, where
neural line conduction resembles electrical conduction in engineering conductors
at much higher frequencies (at frequencies 108 and more times
the neural frequencies). Many of the "revolutionary" results
in neuroscience are consistent under S-K (Appendix 2). Under S-K, neurons
and groups of neurons are much more adapted for information processing
than the same neurons would be under Kelvin-Rall.
To appreciate that, we need to start with a simple account of neuronal
information processing. A typical neuron in the cerebral cortex, the proverbial
gray matter, receives input from a few thousand neurons and, in turn, passes
on messages to a few thousand other neurons (Fig. 1). These connections
are hard wired in the sense that each connection is made by a dedicated
wirea1, the axon, so that, unlike processors in a computer network,
there is no competition for communication bandwidth.
a1) Under S-K, both input and output of the neurons may occur by
inductive coupling as well as direct conduction. When dendritic sections
or spines are in the high transmission mode of S-K, neurons not directly
connected can "communicate" by this inductive coupling.
Apart from the axons, there are three principal components of a neuron.
The cell body (or soma); dendrites, which are short, branched extensions
from the cell body, and which in the traditional view simply receive stimuli
from other neurons and pass them on to the cell body without much further
processing; and synapses, the specialized connections between two neurons.
Synapses are of two types, excitatory and inhibitorya2. An
excitatory synapse will slightly depolarize the electrical potential across
its target cell, while an inhibitory input will hyperpolarize the cell.
If the membrane potential at the cell body exceeds a certain threshold
value, the neuron generates a millisecond-long pulse, called and action
potential or spike. (Fig.2, overleaf). Otherwise, it remains silent. The
amount of synaptic input determines how fast the cell generates spikes;
these spikes are in turn conveyed to the next target cells through the
output axon. Information processing in the average human cortex would rely
on the proper interconnections of about 4 x 1010 such neurons
in a network of stupendous sizeb2.
a2) Under S-K, both excitatory and inhibitory synapses can also produce
local changes in G that generate impedance mismatch reflections (and logical
switching, including the trimming of resonant dendritic structures.) Changes
in membrane resistance due to the opening state of populations of channels
along a line can switch conduction properties from high dissipation properties
essentially like those of Kelvin-Rall, to very low dissipation properties
adapted to resonance.
b2) Under S-K, information processing in cortex relies on BOTH direct
interconnections and the inductive coupling of switched, tunable, adaptive
resonant structures.
In 1943, McCullough and Pitts showed that this (direct connection) view
is at least plausible. They made a series of simplifications that have
been with us ever since. Mathematically, each synapse is modelled by a
single scalar weight, ranging from positive to negative depending on whether
the synapse is excitatory or inhibitory. As a whole, the neuron is represented
as a linear threshold element; that is, the contributions of all the synapses,
multiplied by their synaptic weights, add linearly at the cell body. If
the threshold is exceeded, the neuron generates a spike. McCollough and
Pitts argued that, with the addition of memory, a sufficiently large number
of these logical "neurons," wired together, can compute anything
that can be computed on any digital computera3.
a3) McCullough and Pitts argued that "everything could
be calculated" according to their suggested pattern. They did not
show it. Major difficulties with the M & P case, in addition to the
programming problem Koch treats below, concern speed, dissipation of information
in neural lines (particularly when phase distortion is considered), and
huge semirandom differences in transfer time due to the variable length
of the connecting lines, that would be expected to smear out most or all
of the fast logics that could be proposed. To an engineer who traces logic
step-by-step, these all appear to be crushing difficulties. A difficulty
that has gotten more attention than the others is the combinatorial explosion.
If N is "sufficiently large" then N! is prodigious, a problem
that has remained intractable in machine modelling from the start. For
instance
(65! = 8.24x1090 66!=5.44x1092 67!=3.64x1094)
Under S-K there is inductive coupling of resonant "brain wave"
signals that may contact or coordinate large numbers of neurons in broadcast
mode, in addition to conductive coupling. Information content in resonant
mode is huge, because the resonant properties predicted for known anatomy
and physiology are very selective. The analogy to radar sending and receiving
appears to be close. Resonant bandwidth of spines in the on state should
typically be less than .001 Hz, with magnification factors, Q's, of 104
and higher. The combination of on-state spines, dendritic sections, and
synapses seems to be inherently arranged in a manner adapted to send (or
receive) frequency-time signatures. Such signatures sent (in the proper
phase relation with other oscillations) by means of an A.P. excitation
will only be received in resonance by near-exactly tuned spine-dendritic
section-synapse structures. "Brain wave" oscillation have long
been observed. Because of inductive resonant coupling, line dissipation
and transfer time dissipation constraints become much less important under
S-K than in conduction path constrained models.
Under S-K, resonant interactions can occur contacting many (millions)
of (reactive and nonreactive) neurons simultaneously. An analogy is the
set of logical interactions that can occur among a population of people,
all able to both broadcast and receive radio signals, where some may be
tuned to the same wavelength and some not. The number of interactive combinations
in such an interacting set is prodigious, and the coordinations possible
very detailed. Under S-K, the neural network looks adapted for sending
and receiving signals in the frequency domain. Frequency selectivity of
dendritic spines and spine assemblies is inherently high (with predicted
bandwidths often less than .001 Hz and predicted Q's above 1000). For such
a system, a prodigious amount of information flow can occur by inductive
means, with little or no crosstalk between many frequency-defined "bands".
Under these conditions, the combinatorial limitation of neural nets that
require connection essentially disappears.
Because resonant communication and processing is inherently a massively
parallel process, it avoids the limitations of Feldman's "100 step
rule."
Resonant processing is well adapted to "list reading" problems
such as the lexical access problem, and can handle these in massive parallel,
rather than sequentially. A major reason for rejecting "symbol processing
AI" has been the inherent slowness and "anatomical implausibility"
of the list-reading steps that AI algorithms so often contain. That constraint
is removed by resonant access and processing, which appears to fit anatomy,
and which is very fast.
Significant as their (McCollough and Pitts) result was, it left some
major questions unanswered. Most importantly, how is such a network set
up in the absence of an external programmer? A network of these abstract
neurons has to be programmed from the outside to do any particular job,
but the brain assembles by itself. Clearly, the information needed to provide
a unique specification of the 2 x 1014 synapses in human cerebral
cortex cannot possibly be encoded in the genome. Moreover, a key feature
of biological nervous systems that radically distinguishes them from present-day
computers is their ability to learn from previous experience, presumably
by adjusting synaptic weightsa4. Finally the properties of real
neurons are much more elaborateb4 and variable (Fig 2b) than
the simple and reliable units at the heart of McCullough and Pitts's neural
networks and that of their progeny.
a4. the presumption that memory consists of synaptic weights and
visible connections, and no more, was largely made in default of other
physically testable, sensible alternatives. Under S-K, with its switched,
bimodal transmission, both spines and dendritic sections have properties
adapted to memory when combined with known channels and synaptic structures,
including especially resonance. Under S-K, the information manipulating
power of synapses and channels is MUCH MORE than under current theory.
(For example, a single channel can switch a spine from "on" to
"off" - (from a Q>10,000 state to a Q<10 state. When
transmission under S-K is in the high dissipation mode, S-K and current
theory produce almost the same results.
b4. Spines are an important example of that elaboration. Under S-K,
dendritic spines, if in an all-channel closed state, appear to be very
high Q resonant elements that may have LRC and column resonance modes that
both have Q's of 10,000 or more, with resonant frequencies sensitive to
geometry. With channel opening, these spines go to an "off" state
of negligible resonance. Spines appear to be excellent memory components
in the frequency domain, particularly in interaction with synapse switched
dendritic sections with specific properties (that may be connected to other
spines.) Means to change spine geometry as a function of resonant history
can be learning means.
A possible mechanism addressing the need for self-programming was postulated
a few years later by Donald Hebb: "When an axon on cell A is near
enough to excite cell B and repeatedly or consistently takes part in firing
it, some growth process or metabolic change takes place in one or both
cells such that A's efficiency, as one of the cells firing B, is increased."
Here, according to the principle known as synaptic plasticity, the synapse
between neuron A and neuron B increases its "weight" if activity
in A occurs at the same time as activity in Ba5
a5. Here is an analogous "neoHebbian" statement that may
apply to on state spines and dendritic sections in the frequency domain.
"When a spine-dendrite assembly on cell A sends a frequency
that is near enough to the resonant frequency of a spine-dendrite assembly
on cell B so that the cell B spine is excited, and this resonant coupling
occurs many times, some chemically mediated shaping process may occur to
change the resonant frequency of the spine on Cell B so that it becomes
MORE sensitive to the Cell A frequency signal."
This learning can be a selective process because spine resonant bandwidth
may be less than .001 Hz.
Much of the excitement in the neural network revolution of the 1980's
was triggered by the discovery of learning rules for determining the synaptic
weights using variants of Hebb's rule of synaptic plasticity. These rules
allow the synaptic weights to be adjusted so that the network computes
some sensible function of its input - it learns to recognize a face, to
encode eye position or to predict the stock market, for example.
Underlying these developments was the view that memory was stored in
the synaptic weights. Changing connection strength can make the network
converge to a different stable equilibrium, equivalent to recalling a different
memory (associative memory, ref 3). Experimentally, the best studied aspect
of increasing connection strength is long-term potentiation, which can
be categorized as an increase in synaptic weight lasting for days or even
weeks. It is induced by simultaneous activity in the pre-and postsynaptic
terminals (Fig.1), in agreement with Hebb's rule. Of more recent vintage
is the discovery of a complementary process, a decrease in synaptic weight
termed long-term depressiona6.
a6. Both LTP and LDP become MORE IMPORTANT and more powerful information
storage processes under S-K, because they can modulate frequencies, and
because they can change firing thresholds of resonant structures.
These neural-network models assumed that, to cope with the apparent
lack of reliability of single cells, the brain makes use of a "firing
rate" code. Here, only the average number of spikes within some suitable
time window, say a fraction of a second, matters. The detailed pattern
of spikes was thought to be largely irrelevant (Fig, 2b). This hypothesis
was supported by experiments in which monkeys were given the task of discriminating
a pattern of moving dots amid background noise. The monkey's performance
could be statistically predicted by counting spikes in single neurons within
visual cortex, implying that all of the information needed to perform the
task is available from the average firing rate alone. Further precision
could in principle be obtained by averaging the firing rates over a large
number of neurons (a process known as population coding), without any need
to invoke the precise timing of individual spikes as a source of informationa7.
a7. Under S-K, many neural components (particularly spines, and
lengths of dendrites switched between pairs of synapses) are inherently
high precision components in resonant mode. That is useful from an
"engineering" perspective. Averaging is a poor substitute for
component precision in engineering practice for good reasons. Averaging
takes time and space, and generally requires very high accuracy components
within the averager apparatus. Typically, even if time is not at a premium,
averaging approaches take many more precision components than accurate
transduction means would have taken in the first place.
A snapshot of the "standard view" of information processing
in the brain, say as of 1984, would be based on simple, linear-threshold
neurons using firing-rate code that waxes and wanes over hundreds of milliseconds
to communicate information among neurons. Memory and computation are assumed
to be expressed by the firing activity of large populations of neurons
whose synaptic weights are appropriately set by synaptic learning algorithms.
But since then neuroscience has undergone a phase of exponential growth,
with progress in three separate areas - in the study of dendrites, of spikes
and their timing, and of synaptic plasticity.
ACTIVE DENDRITES
It was Ramon y Cajal who, in the late nineteenth century, first revealed
the intricacies and complexities of real neurons. He showed that a prototypical
neuron, say a cortical pyramidal cell as shown in Figs 1 and 3, has an
extended dendritic tree which is scores of times larger in surface area
than the cell body. It has become clear that dendrites do much more than
simply convey synaptic inputs to the cell body for linear summation. Indeed,
if this is all they did, it is not obvious why dendrites would be needed
at all; neurons could be spherical in shape and large enough to accommodate
all the synaptic inputs directly onto their cell bodies. A few neurons
do follow this geometrical arrangement but the vast majority are more like
the cell shown in Fig. 3, with an extended dendritic tree. This is where
many of the synaptic inputs to the cell are received, but much of the membrane
area is still devoid of synapses; so the function of the elaborate structure
cannot simply be to maximize the surface area for synaptic contact.
Dendrites have traditionally been treated as passive cables, surrounded
by a membrane which can be modelled by a conductance in parallel with a
capacitor. When synaptic input is applied, such an arrangement acts as
a low-pass filter, removing the high frequencies but performing no other
significant information processinga8. Dendrites with such passive
membranes would not really perturb our view of neurons as linear threshold
unitsb8.
a8. Under S-K, the differential equation of conduction (in channel-closed,
or low G mode) is of the same form as the standard electrical transmission
equation. Transmission line effects seen in the gigahertz and higher range
(including resonance and very sharp impedance mismatch switching) are reproduced
in the neurological frequency range. Under S-K the dendrites that "appeared
to the neuroanatomist Ramon y Cajal to be elaborate receiving antennae,
studded with thousands of synapses(2)"
are just that and more.
The dendrites are not "just antennae" - they are active
components. Under S-K, the dendrites contain a great deal of spatially
detailed switching capacity, with each synapse capable of impedance mismatch
switching, with channels in the aggregate switching transmission from a
very high dissipation to a very low dissipation conduction mode, and with
the spines as very high Q coupled, switched resonators at many places on
the dendrite. This dendrite is a receiver, a sender, and a processor
of information by inductive means, and also transmits by conduction in
the long-studied ways.
b8. Under S-K, dendrites are much more complicated than linear threshold
units even if they are passive (in the sense of acting without any openings
or closings of channels or synapses) because of resonant effects. When
synaptic and channel activity is added, the complication (and information
handling and learning capacity) greatly increases.
But dendrite membranes are not passive - they contain voltage-dependent
membrane conductances. These conductances are mediated by protein complexes
that allow charged ions to flow across the membrane. As long ago as the
1950's, Hodgkin and Huxley showed how the transient charges in such conductances
generate and shape the action potentiala9. But it was assumed
that they are limited to the axon and the adjacent cell body. We now know
that many dendrites of pyramidal cells are endowed with a relatively homogeneous
distribution of sodium conductances as well as a diversity of calcium membrane
conductancesb9.
a9. Hodgkin and Huxley were very clear about whole-axon action potentials.
They were not so clear about how their action potential propagated
down a neural line. The Kelvin equation is a standard electrical engineering
equation (with L negligible) and the behavior of the (Kelvin-Rall) RC line
shows conduction velocity that varies as the square root of (fourier
component) frequency(3). Whether that
RC line is propagating passively or in a channel amplified mode, the observed
coherence of A.P. waveform (which has many very different fourier components)
during propagation seems impossible. The high frequency components should
outrun the low frequency components, and they do not. With S-K, that difficulty
is removed, because different fourier components propagate at the same
velocity. Calculated and observed A.P. propagation velocities seem to match
predictions under S-K. The logic of the action potential that Hodgkin
and Huxley set out in most detail fits S-K well, but is incompatible with
the Kelvin-Rall model now used.
b9. Even if difficulties due to differential velocity of different
fourier components under Kelvin-Rall is set aside, there is another major
difficulty. Any heterogeneity of channel conductances offers difficulty
for A.P. propagation under Kelvin-Rall, because that model depends on a
continuous and differentiable amplification to compensate for the inherently
very dissipative passive mode of propagation under Kelvin-Rall. Very uniform
channel densities are also needed to avoid impedance reflection effects.
Observations seldom seem to show totally smooth channel densities along
dendrites. There is more reason to believe heterogeneity is the rule -
indeed Zecevic(4) showed stunning heterogeneity,
with "multiple spike initiation zones" in an axon that showed
rather conventional A.P. behavior. These results are inconsistent with
Kelvin-Rall, but are consistent with S-K, which has a high effective line
inductance and far less dissipation than predicted under Kelvin-Rall.
What is the function of these active conductances? In a passive cable
structure, synaptic input to the more distant regions of the dendritic
tree (as the to of Fig.3) would quickly saturate, delivering only a paltry
amount of electric current to the spike initiating zone far awaya10.
But it turns out that synaptic input to this part of the tree is sufficient
to elicit somatic action potentials, and the likely explanation (supported
by computer models) is that calcium and potassium membrane conductances
in the distant dendrites can selectively amplify this inputb10.
a10. Under S-K, dissipation will be much less than in Kelvin-Rall,
but still important enough to provide good reason for amplification.
b10. Under S-K, calcium and potassium conductances can still amplify
as argued in Bernander, Koch, and Douglas. However, there is somewhat less
need for amplification, and the conductances can be logically active in
other ways.
Voltage-dependent conductances can also subserve a specific nonlinear
operation, multiplication. In a passive dendritic tree, the effect of two
synaptic inputs is usually less than the sum of the two individual inputs;
that is, they show saturation. This saturation effect can be minimized
by spreading the synapses far apart. If, however, the dendritic tree contains
sodium and calcium conductances, or if the synapses use a particular kind
of receptor (the so-called NMDA receptor), the inputs can interact synergistically:
now, the strongest response occurs if inputs from different neurons are
located close to each other on a patch of dendritic membrane. Computer
simulations show that such a neuron effectively performs a multiplication;
that is, its firing rate is proportional to the product, rather than the
sum, of its inputs. Multiplication is one of the most common operations
carried out in the nervous system (for example, for estimating motion or
the time-to-contact with an approaching stimulus), and it is tempting to
speculate that this may be achieved through such processes at the cellular
level.
A further development has been the realization that the distribution
of calcium ions within dendrites may represent another crucial variable
for processing and storing information. Calcium enters the dendrites through
the voltage-gated channels, and this, along with its diffusion, buffering
and release from intracellular stores, leads to rapid local modulations
of calcium concentration within the dendritic tree. The concentration of
calcium can, in turn, influence the membrane potential (through calcium-dependent
membrane conductances) and - by binding to buffers and enzymes - turn local
biochemical signalling pathways on or offa11.
a11. Under S-K, calcium can switch propagation between a low dissipation
mode and a (Kelvin-Rall like) high dissipation mode by changing g.
It was also Ramon y Cajal who postulated the law of "dynamic polarization,'
which stipulates that dendrites and cell bodies are the receptive areas
for the synaptic input, and that the resulting output pulses are distributed
unidirectionally along the axon to its targets. This assumes that action
potentials travel only along axons: no signal was thought to travel outwards
along the dendrites.
From work on brain slices, however, it seems that this is by no means
the whole story. Single action potentials can propagate not only forwards
from their initiation site along the axon, but also backwards into the
dendritic tree (a phenomenon known as "antidromic spike invasion").
It remains unclear whether dendrites can initiate action potentials themselves.
If they can, such dendritic spikes could support theoretical proposals
that all-or-none logical operations occur in the dendritic tree. The next
step will be to find out whether action potentials can propagate into the
dendritic tree under more natural conditions - that is, using sensory stimuli
in an intact animal.
Thus, it is now evident that the dendritic tree is far more complex
than the linear cable models of yesteryear assumed. Dendrites provide the
substrate for numerous nonlinear operations, and endow neurons with much
greater information-processing capacity than was previously suspecteda12.
a12. Change to S-K, and complexity increases, precision increases,
and information processing ability increases. Resonance-mediated memory,
including spine resonance and synapse switched dendritic section resonance,
becomes available. The dendrites become able to send and receive frequency
coded information over long distances via inductive coupling. The logical
importance of individual synapses and groups of channels is much greater
under S-K than under Kelvin-Rall.
TIMING COUNTS
The second area in which our thinking has changed has to do with the
role of time in neuronal processing. There are two main aspects to this
issue - first, the relationship between the timing of an event in the external
world and the timing of the representation of that event at the single-neuron
level; second, the accuracy and importance of the relative timing of spikes
between two or more neurons.
Regarding the first question, some animals can discriminate intervals
of the order of a microsecond (for instance, to localize sounds), implying
that the timing of sensory stimuli must be represented with similar precision
in the braina13. But this usually involves highly specialized
pathways, probably based on the average timing of spikes in a population
of cellsb13. However, it is also possible to measure the precision
with which individual cells track the timing of external events. For instance,
certain cells in the monkey visual cortex are preferentially stimulated
by moving stimuli, and these cells can modulate their firing rate with
a precision of less than 10 ms (ref 16).
a13. Under S-K, wave propagation (and reflection) in dendritic spines
and dendritic sections is precise enough that single components can be
very accurate timers, particularly for resonant columns detecting and comparing
spike-containing signals. For example stereocilia are 1/4 wave resonant
columns, constructable from 10 Hz to 150,000 Hz, with geometries consistent
with known stereocilia anatomy. With S-K, the "engineering difficulty"
of building a microsecond sensitive timer and interval representation component
is large, but it is a much easier job than would be involved with Kelvin-Rall.
b13. With S-K, averaging may not be necessary. Single component accuracy
should be good enough. (If it isn't, how does averaging help you? You must,
after all make your averaging apparatus out of something real. Averaging
soaks up time and space. The total system has to be very good indeed if
you have to process as much information as a bat does.)
The second aspect of the timing issue is the extent to which the exact
temporal arrangement of spikes - both within a single neuron and across
several neurons - matters from information processing. In the past few
years there has been a resurgence of signal processing and information-theoretical
approaches to the nervous system. In consequence, we now know that individual
neurons, such as motion-selective cells in the fly or single auditory inputs
in the bullfrog, can encode between 1 and 3 bits of sensory information
per strike, amounting to rates of up to 300 bits per second. This information
seems to be encoded using changes in the instantaneous interspike interval
between a handful of spikesa14. Such a temporal encoding mechanism
is within 10-40 per cent of the theoretical maximum allowed by the spike
train variability. This is quite remarkable because it implies that individual
spikes in a single cell in the periphery can carry significant amounts
of information, quite at odds with the idea that neurons are very unreliable
and can only signal in the aggregate. At these rates, the optic nerve,
which contains about one million fibers, would convey between on and a
hundred million bits per second - compare this with a quad-speed CD-ROM
drive, which transfers information at 4.8 million bits per second.
a14. Under S-K, spines and dendritic sections are well adapted to
compare interspike intervals, particularly in high Q column resonant mode
(long spines).
The relative timing of spikes in two or more neurons can also be remarkably
precise. For instance, spikes in neighboring cells in the lateral geniculate
nucleus, which lies midway between the retina and the visual cortex, are
synchronized to within a millisecond. And within the cortex, pairs of cells
may fire action potentials at predictable intervals that can be as long
as 200 ms, two orders of magnitude longer than the delay due to a direct
synaptic connection, with a precision of about 1 msa15. Such
timing precision across populations of simultaneously firing neurons is
believed to be a key element in neuronal strategies for encoding perceptual
information in the sensory pathwaysb15.
a15. Under S-K, with inductive coupling via the extracellular medium,
this coordination is exactly what one would expect.
b15. To keep a frequency domain code straight, oscillatory coordination
is extremely desirable so that frequency-temporal messages can be sent
and received at standard cycle times. (Radar technology has many examples
of this sort of signal timing, and so does ordinary language.)
If neurons care so much about the precise timing of spikes - this is,
if information is indeed embodied in a temporal code - how, if at all,
is it decoded by the target neurons? Do neurons act as coincidence detectors,
able to detect the arrival time of incoming spikes at millisecond or better
resolution? Or do they integrate more than a hundred or so relatively small
inputs over many tens of milliseconds until the threshold for spike initiation
is reached? (Ref 23, Fig 2a) These questions continue to be widely and
hotly debateda16.
a16. Under S-K, dendrite-spine assemblies are well adapted to receive
temporal code via resonant inductive coupling. Decoding by resonance is
inherent when tuning occurs. A significantly strong signal can fire action
potentials. Signal resolution can be considerably better than 1 millisecond.
Averaging is not necessary.
SYNAPTIC PLASTICITY
Back-propagating action potentials are puzzling if considered solely
within the context of information processinga17. But they make
a lot of sense is seen as "acknowledgement signals" for synaptic
plasticity and learning. A Hebbian synapse is strengthened when pre and
postsynaptic activity coincide. This can occur if the presynaptic spike
coincides with the postsynaptic spike that is generated close to the cell
body and spreads back along the dendritic tree to the synapse. A new and
beautiful study shows that the order of the arrival time between the presynaptic
spike and the back-propagated postsynaptic spike is critical for synaptic
plasticity.
a17. In a system with switched resonance, backpropagating a.p.'s
make sense for processing, as well as for learning.
If the presynaptic spike precedes the postsynaptic spike, as should
occur if the first participates in triggering the second, then long term
potentiation occurs - that is, the synaptic weight increases. If, however,
the order is reversed, the synaptic weight decreases. So sensitive is this
sequence that a change of timing of as little as 10 ms either way can determine
whether a synapse is potentiated or depresseda18. The purpose
of this precision is presumably to enable the system to assign credit to
those synapses that were actually responsible for generating the postsynaptic
spike.
a18. In resonance, which is to be expected under S-K, the notion
that a phase difference may either magnify or reduce a response (or, by
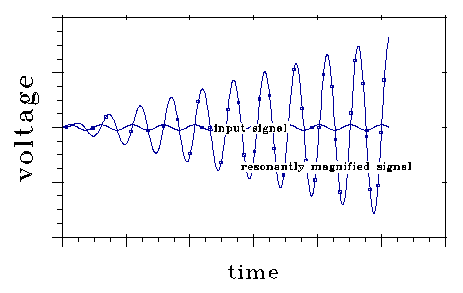
a small extension, a weight) comes naturally. Here is a curve showing resonant
magnification, with the perturbation (input) signal phased so that resonance
builds up. It, at some time t, input signal phase shifted 180 degrees,
the resonant signal would decrease at the same rate. Around null,
a shift of a few degrees makes the difference between rather rapid resonant
signal increase and rapid decrease. FM radio operates on this principle.
This property of a resonant system can be used to increase its ability
to carry and process information.

These (time sensitive) experiments come at a fortuitous time, for theoretical
work has begun to incorporate asymmetric timing rules into neural network
models. The attraction of doing so is that it allows the network to form
associations over time, enabling it to learn sequences and predict events.
It is therefore gratifying to find that synapses with the required properties
do indeed exist within the brain.
In the past year there has also been the emergence of a new way of thinking
about short-term plasticity, one that complements the view of long-term
synaptic changes for memory storage. This has come about by joint experimental-theoretical
work (refs 26, 27, see overleaf), suggesting that individual synapses rapidly
adapt to the presynaptic firing rate, primarily signalling an increase
or a decrease in their input. That is, synapses continuously adapt to their
input, only signalling relative changes, which means that the system can
respond in a highly sensitive manner to a constantly and widely varying
external and internal environmenta19. This is entirely different
from digital computers that enforce a strict segregation between memory
(onboard cache, RAM, or disk) and computation. Indeed they are carefully
designed to avoid adaptation and other usage dependent effects from occurring.
a19) Under S-K, this might better read "synapse-dendritic
section assemblies continuously adapt to their input, so that they
only signal relative changes, which means that the system can respond in
a highly sensitive manner to a constantly and widely varying external and
internal environment." The rephrasing would maintain the thrust of
the logic, and would be consistent with observations.
Interestingly, single-transistor learning synapses - based on the floating
gate concept underlying erasable programmable ROM digital memory - have
now been built in a standard CMOS manufacturing process. Like biological
synapses, they can change their effective weight in a continuous manner
while they carry out computations. Floating-gate synapses will greatly
aid attempts to replicate the functionality of nervous systems by the appropriate
design of neuromorphic silicon neurons using analog very-large-scale-integrated
(VLSI) circuit fabrication technology.
HYBRID COMPUTER
Overall, then, current thinking about computation in the nervous system
has the brain as a hybrid computer. Individual nerve cells convert the
incoming streams of digital pulses into spatially distributed variables,
the postsynaptic membrane potential and calcium redistribution. This transformation
involves highly dynamic synapses that adapt to their inputsa20.
a20) Under S-K, "highly dynamic synapses" would be interpreted
as "highly dynamic synapse-dendritic section assemblies."
Information is then processed in the analog domain, using a number of
linear and nonlinear operations (multiplication, saturation, amplification,
thresholdinga21) implemented in the dendritic cable structure
and augmented by voltage dependent membrane and synaptic conductances.
The resulting signal is then converted back into digital pulses and conveyed
to the following neuronsb21. The functional resolution of these
pulses is in the millisecond range, with temporal synchrony across neurons
likely to contribute to coding. Reliability could be achieved by pooling
the responses of a small number (20-200) of neuronsc21.
a21. Also resonance under S-K.
b21. Under S-K, conduction and inductive coupling through the intercellular
medium both occur.
c21. Under S-K, reliability and precision in resonant or time delay
mode is inherently high, and averaging is unnecessary or less necessary.
And what of memory? It is everywhere (but can't be randomly accessed).
It resides in the concentration of free calcium in dendrites and the cell
body; in the presynaptic terminal; in the density and exact voltage-dependency
of the various ionic conductances; and the density and configuration of
specific proteins in the postsynaptic terminalsa22.
a22. Under S-K, much more specific memory than that described above
follows near-inherently from anatomy. The resonant memory structures are
more adapted to learning and to rapid readout of memory from large volumes
of cells, for jobs like lexical access. Spines are very high Q resonant
objects (with both LRC and column resonance modes) that are channel switched
on and off. Plausible modifications seem simply adapted to let spines "learn"
different pitches through a mechanism like LTP. Spine-dendrite-synapse
assembly states seem adaptable to store temporal signatures of high information
content in resonance, and to respond to such coded stimuli in stereotyped
ways (generating action potentials). With action potentials serving to
excite resonant dendritic structures, temporal signatures may also be "broadcast"
into the extracellular medium. This view fits the very sharply resonant
data of David Regan (Appendix). Regan's fast FFT EEG measurements under
evoked conditions routinely measured resonantly organized signals in sharp
patterns with many peaks with bandwidths as tight as .002 Hz or tighter.
Regan's data may be interpreted to show the combined effect of large populations
of sharply resonant spines, and match model predictions under S-K.
It appears that all the learning modes suggested by Koch and
others also continue to be available under S-K.
Only very little of this complexity is reflected in today's neural network
literature. Indeed, we sorely require theoretical tools that deal with
signal and information processing in cascades of such hybrid, analog-digital
computational elements. We also need an experimental basis, coupled with
novel unsupervised learning algorithms, to understand how the conductances
of a neuron's cell body and dendritic membrane develop in time. Can some
optimization principle be found to explain their spatial distribution?
As always, we are left with a feeling of awe for the amazing complexity
found in nature. Loops within loops across many temporal and spatial scales.
And one has the distinct feeling that we have not yet revealed every layer
of the oniona23. Computation can also be implemented biochemically
- raising the fascinating possibility that the elaborate regulatory network
of proteins, second massagers and other signalling molecules in the neuron
carry out specific computations not only at the cellular but also at the
molecular level.
a23. Under S-K the old data remains important, and much of the old
theory does as well. (One of the modes of S-K is nearly identical to Kelvin
Rall.) But the information processing capacity of individual dendrites
and neural tissue becomes much greater under S-K, and the capacities of
brain become somewhat more comprehensible.
ARTICLE INSERT: THE ADAPTABLE SYNAPSEa24
In short-term synaptic depression, the postsynaptic response to a regular
train of presynaptic spikes firing at a fixed frequency f gradually
lessens. The response of the first spike might be large, but subsequent
responses will be diminished until they reach a steady state (expressed
in terms of A, the fractional reduction in postsynaptic effect). This is
shown here (part a) for presynaptic spikes at 40 Hz frequency (data from
ref 26).
This depression generally recovers within 0.1 to 0.5 s. For firing rates above 10 Hz, A is roughly inversely proportional to the firing frequency. In other words, within a few hundred milliseconds the synapse will have adapted to the presynaptic firing with a response roughly independent of the firing rate (due to the inverse relationship between A and f). If the synaptic
consequence, the transient change in the postsynaptic response will be
proportional to the relative change in firing frequency. This is demonstrated
in computer simulations in which the presynaptic firing rate of a couple
of hundred such synapses converging onto a model neuron is increased fourfold
(part b - the increases are from 25 to 100 Hz on the left and from 50 to
200 Hz on the right; data from ref. 27).
Even though the final input rate is twice as high on the right side
as on the left, the firing rate of the neuron is roughly the same. This
is because the fractional increase - relative to the background rate -
is the same in both cases. This form of short-term depression in synaptic
strength therefore enables synapses to respond to relative changes in firing
rates rather than to absolute rates.
a24) Under S-K, none of the interpretations Koch discusses in this
insert need change (one of the modes under S-K is nearly identical to Kelvin-Rall).
However, under S-K adaptations may also be by means of dendritic section
(or spine) synapse assemblies, rather than synapses alone. For such assemblies,
the precise timing of wave propagation, and the sensitivity of S-K to local
values of g could produce rapid, sensitive adaptation.
Appendix 1. Resonance
in neurons, as measured, and as calculated by S-K theory.
David Regan has measured brain magnetic fields (MEG) and scalp
voltages (EEG) during evoked stimulation. Figs 9 and 10(5)
show some of Regan's measurements using his zoom FFT technique.
Fig. 9
The caption for this figure reads:
"The stimulus was a homogeneous patch of light flickering at F1
superimposed on a second patch flickering at F2. The EEG was
analyzed by nondestructive zoom FFT at resolution of 0.0039 Hz. Recording
duration was 320 seconds. The section contains 12,000 lines over a bandwidth
of .5 - 49.5 Hz. The steady-state evoked potential consists of discrete
frequency components whose bandwidths are less than 0.0039 Hz. . . . "
The data have characteristics often seen in resonant systems with very
low damping, with sums and integer multiples of the stimulus."
Figure 10 shows evidence of bandwidths narrower than bin widths of .0019
Hz. The 4F component is shown. Bandwidths of the peaks measured in Fig
9 may have been no wider than this.
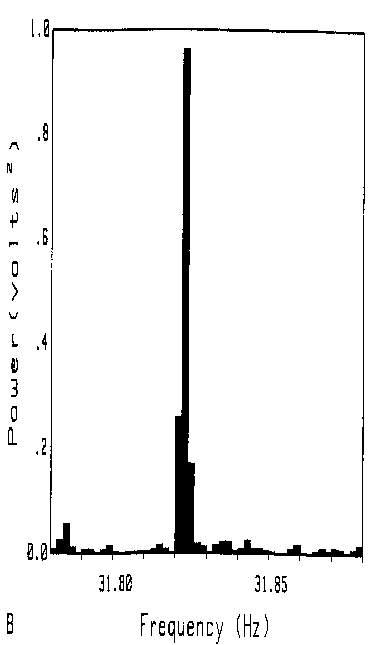
Fig. 10
These sharp, high information content patterns, with stimuli frequency
multiples organized roughly as shown, show that brain includes sharply
resonant components, and shows that these components are coupled with very
small lags and with small damping. The bandwidths Regan measures are too
sharp by at least an order of magnitude to be produced by membrane channel
activity.
The pyramidal cells in brain, interpreted according to the S-K theory,
should behave in a manner that generates the kind of behavior that Regan
measured. Conduction lines have very low distortion. Effective line inductances
are far greater than those predicted by Kelvin-Rall - high enough so that
neurons can be inductively coupled via the intercellular medium, which
conducts millions of time faster than line conduction speed. The dendritic
spines have the sharply resonant requirements Regan's data appears to require.
Resonance:
Resonance is logically interesting. Enormous resonant magnifications
of tightly selected signals are possible. In this sense, resonant systems
can function as highly selective amplifiers. This fact is a foundation
of communication technology. Radio and television offer familiar examples
of resonant selectivity. Radio and television receivers exist in an electromagnetic
field consisting of a bewildering and undescribable variety of electromagnetic
fluctuations. Reception occurs because the resonant receiver is selective
for a specific frequency at a high degree of phase coherence. Signals off
frequency are not significantly detected, and "signals" of random
phase that are on frequency cancel rather than magnify in resonance. Radar
receivers also operate on the principle of resonance. Other examples are
our telephone system and cable television system, each organized so that
a multiplicity of different signals can be carried in physically mixed
form over the same conduits. These "mixed" signals can be separated
and detected with negligible crosstalk by resonant means.
Electrical resonance can store up energy in an oscillation having a
peak voltage Q times the oscillating voltage of the exciting disturbance.
Resonant systems may all be described in wave propagation terms, and many
can also be treated in lumped terms. The LRC oscillator common in differential
equation textbooks is an example of a resonant system described in lumped
terms.
The International Dictionary of Applied Mathematics explains
inductance-resistance-capacitance (LRC) series resonance as follows, and
describes behavior generally characteristic of resonance. The "coil"
is a lumped inductance, the "condenser" is a lumped capacitance,
and "j" is the square root of -1.
. . . In an a-c circuit containing inductance and capacitance in
series ... the impedance is given by
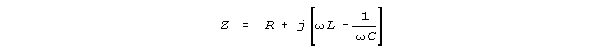
![]()
![]()
L is the inductance, and C is the capacitance. It can be readily
seen that at some frequency the terms in the bracket will cancel each other,
and the impedance will equal the resistance alone. This condition, which
gives a minimum impedance (and thus a maximum current for a fixed impressed
voltage) and unity power factor is known as series resonance. Where the
resistance is (relatively) small the current may become quite large. As
the voltage drop across the condenser or coil is the product of the current
and the impedance of that particular unit, it may also become very large.
The condition of resonance may even give rise to a voltage across one of
these units that is many times the voltage across the whole circuit, being,
in fact, Q times the applied voltage for the condenser and nearly that
for the coil. This is possible since the drops across the coil and condenser
are nearly 180 degrees out of phase, and thus almost cancel one another,
leaving a relatively small total voltage across the circuit . . .(6)
Fig 11 shows how the voltage oscillation stored in a resonant system
grows when it is driven by a input signal at its resonant frequency. The
growth shown depends on stimulus phase, a fact on which FM transmission
depends. If, at some time, the input signal voltage shown were to shift
phase 180 degrees, the resonant voltage would decrease as fast as it is
shown increasing here.

![]()
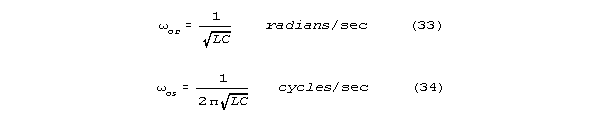
The resonant amplification factor, Q, achieved after time to
equilibrium(7), is:
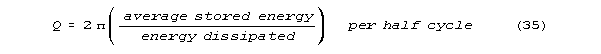
For an LRC resonator, Q is
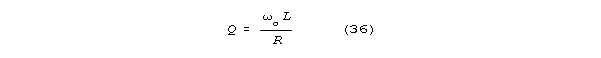
High Q's are prized in information processing systems, partly because
bandwidth (the frequency difference between the half power points on a
resonance curve) is inversely related to Q according to the formula:
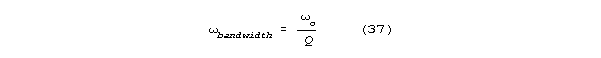
For spines, Q's in the tens of thousands are possible.
Columns can also be sharply resonant. Columns (transmission lines) of
1/4 and 1/2 wavelength have been used as resonators in musical instruments
for many centuries. More recently, column resonance has been used with
precision in the radar and communication fields. Well terminated lengths
of neural passage that are sharply open (short circuited) at both ends
are resonant when there length is exactly 1/4 of wavelength. For column
length lc:
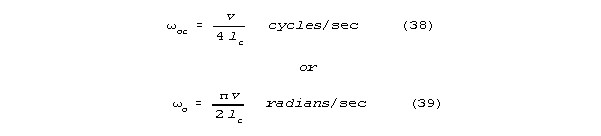
and integer multiples of resonant frequency, omegao. A well terminated length of neural
passage that sharply closed on one end will be resonant at
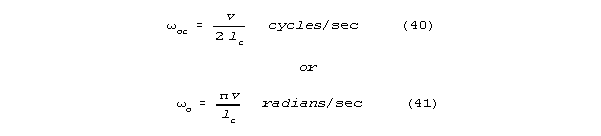
and integer multiples of these frequencies.
For a neural line in the constant velocity regime:
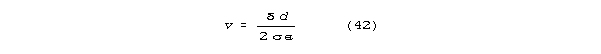
As a 1/4 wave resonant column of length lc:
 .
.
Q of the column resonator will be inverse with attenuation per 
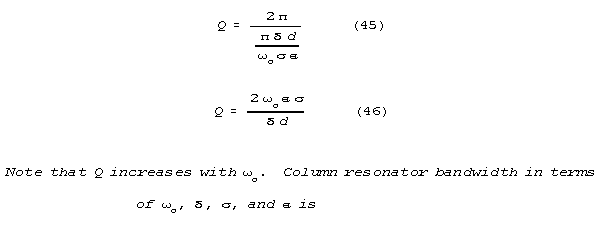
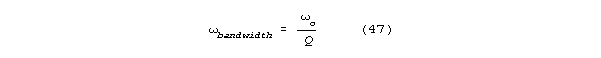

Column resonators are more powerful information handling devices
than lumped resonators of the same Q, because they magnify and store
repeating WAVEFORMS that fit as standing waves within them. (For this reason,
a wind instrument or pipe organ wave form can be much more complicated
than a sine wave.) In contrast, an LRC resonator stores a sine wave.
The brain appears to be a resonant system, and if it may be judged by
the Q's it shows, a very capable one. Considering Regan's frequencies
of 7-46 Hz, and setting bw at .0019 Hz, we calculate Q's
of 3680 to 24,200 for the ensembles that represent frequency peaks. These
are very high calculated ensemble Q's, higher than the Q's
of even the best nonsuperconducting tuned circuits. Individual resonator
Q's must be higher still. Regan's measurements give upper bounds
on bandwidths. From neuroanatomy, the candidate resonant structure seems
plain - the dendritic spines. There are about 1013 dendritic
spines in brain, and, based on the values of effective inductance predicted
here, the spines appear to be resonant structures with very high resonant
amplification factors (Q's).
SPINE ANATOMY AND SPINE RESONANCE:
Fig 12(8) shows camera lucida drawings
of a neuron body and proximal dendrites of a rat hippocampal (CA1) neuron
showing common spine types: thin, mushroom shaped, and stubby. The "thin"
type is the commonest in cortex (about 70%), and can be considered as both
an LRC element and a column resonant element. In LRC mode, the bag and
shaft have a lumped capacitance, and shaft inductance and resistance are
considered in lumped form. In column mode, the shaft is a column open at
both ends, with a termination correction.
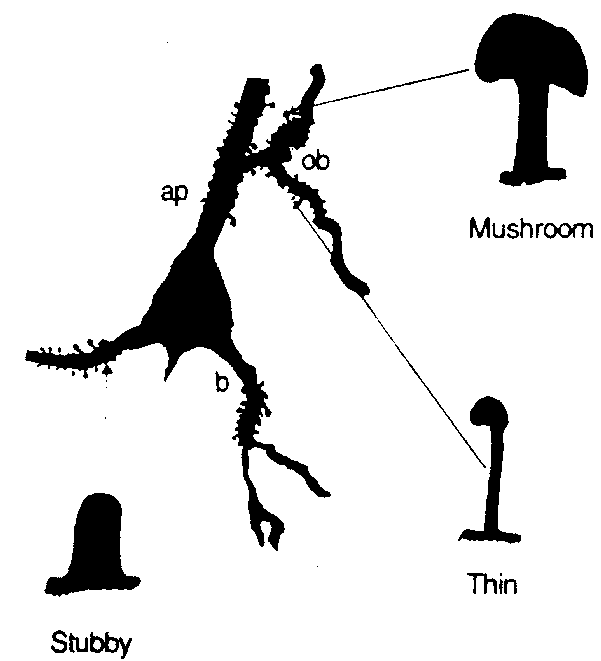
Figure 13 shows an electrical model of a thin spine. In the scaled figure,
4/5 of spine capacitance is in the bag section.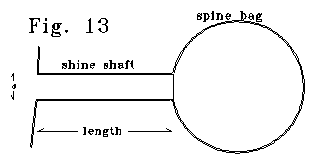
The "spine" of Fig. 13 can be modelled as an LRC resonant
system. Capacitance is the capacitance of the "bag" section,
plus half the capacitance of the shaft section. The shaft has resistance
of R, and an effective inductance of Ledelta x. The Le/R
ratio is inverse with diameter. Different bag sizes for the same shaft
size yield different LC products, and different resonant frequencies. Q,
radian frequency, and bandwidth, for the LRC case are:
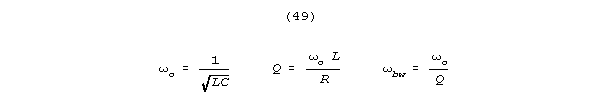
In the model, bandwidth is proportional to diameter. Let's arbitrarily chose a shaft diameter of .1 micron, shaft length of
.5 micron, interspine medium conductance of 110 ohm-cm, membrane capacitance
of 1 microfarad/cm2, and zero membrane leakage, g. Holding
these values, and varying bag size, yields the following relation between
frequency and Q.
Q = o(910)
For Regan's measured frequency range of 7-45 hz (44-283 radians/sec,)
Q's between 40,000 and 257,000 are estimated. Regan's data correspond
to Q's about a decade smaller, between 3,680 and 24,000 over that
same 7-45 Hz frequency range. This is an acceptable fit because:
Regan must have measured ensemble properties, not properties of single
neural elements.
Regan's setup could have detected no tighter bandwidths than he did
detect. and
Within the constraints of biological knowledge, we could have guessed other values of the parameters to come closer to Regan's values (or even to match them.)
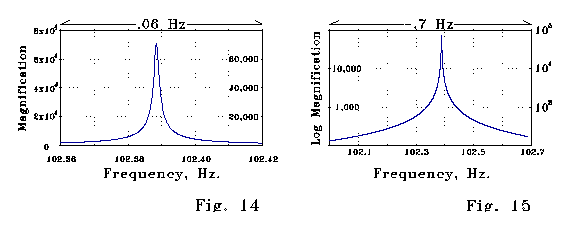
Figs 14 and 15 below show steady-state magnification of a signal as
a function of frequency calculated for the LRC spine model of Fig 13. The
peak magnification factor is about 70,000. Note the sharpness of the magnification
as a function of frequency.
The model spine of Fig 13 would also have a column resonance mode. Spine
column resonant frequency will be approximately

and could estimate column resonant Q from equation () as
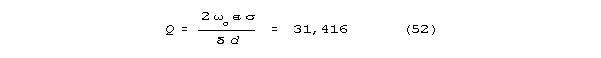
Electrical compliance of the bag would shift these resonant frequencies
and Q's somewhat from the simple 1/4 wave column calculation set out above,
but the correction would involve details that can be considered elsewhere.
Referring again to Regan's data, the brain has many (about 1013)
spines. If spine resonant frequencies are widely distributed, and some
reasonable fraction of the dendritic spines are in the high Q state,
one would expect fixed frequency stimuli, such as Regan supplied, to yield
the sort of excitation curves that Regan observed. Coupling of the spines
would be via the very rapid conduction of the extracellular medium, not
via conduction along dendrites or axons.
SPINE SWITCHING:
Spines are adapted for off switches. A single membrane channel can turn
off spine resonance. This may be useful
because otherwise, voltage buildups sufficient to break down the dielectric
of the spine bags, with consequent spine destruction, might occur(9)
and
because binary switching is useful in information processing.
Suppose there is one membrane channel in the bag portion of the spine.
If that channel is open, it acts as a shunt, damping voltage fluctuations
that occur across it. There will be a shunt across the bag membrane. The
spine will have a shunt limited Q, Qdamped. Let Rc
be shunt channel resistance. If Qdamped<<Q, as it will
be for a channel, we can say that:
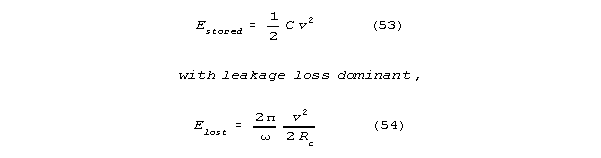
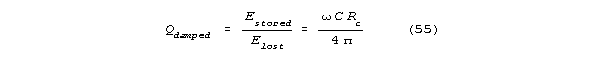
The Qdamped << Q assumption makes sense for reasonable
membrane channel values (between 4 and 400 picosiemens(10).)
Opening of one channel will change a spine from a sharply resonant state,
with a Q in the thousands, to a Q less than 10, a very wide bandwidth state.
A single channel therefore acts as an on-off switch.
Closure:
Evidence and argument has been presented here supporting the position
that current neuroscience data cited by Koch and connected to Koch's article,
combined with the zoom FFT EEG measurements of David Regan, strongly
supports the Showalter-Kline (S-K) passive conduction theory. That theory
appears to permit memory, processing speeds and logical capacities that
are much more brain-like than permitted by the current Kelvin-Rall conduction
model. The annotation of Professor Koch's article was employed here as
a good way to show how S-K theory fits in with what is now known and thought
about neural computation. Regan's data, combined with the data cited
and interpreted by Koch, requires the sort of sharp resonance-like brain
behavior that occurs under S-K, that cannot occur under Kelvin-Rall.
Appendix 2: The S-K transmission equations:
The currently accepted passive neural conduction equations are the standard
conduction equations of electrical engineering, usually written in a contracted
form that discards the terms in L that are negligible in this equation.
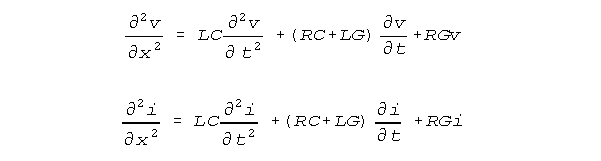
Robert Showalter and Stephen Jay Kline have found that these equations lack crossterms because special restrictions on the use of dimensional parameters in coupled finite increment equations have not been understood(11)
. The crossterms, which can also be derived (within a scale constant)
by standard linear algebra based circuit modelling, are negligible in most
engineering applications. But these crossterms are very large in the neural
context. The Showalter-Kline (S-K) equations are isomorphic to the standard
conduction equations of electrical engineering and are written as follows.
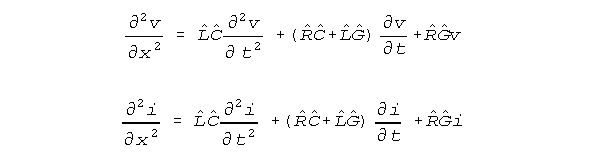
For set values of resistance R, inductance L, membrane
conductance per length G, and capacitance C, these equations
have the solutions long used in electrical engineering. The hatted values
are based on a notation adapted to crossproduct terms. In this notation,
the dimensional coefficients are divided into separate real number parts
(that carry n subscripts) and dimensional unit groups, as follows.
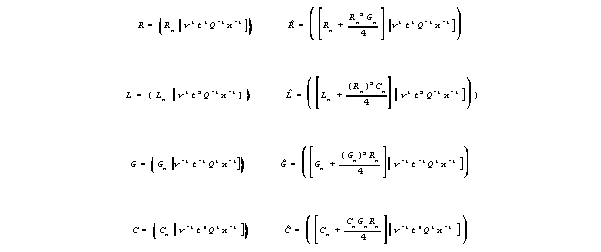
For wires,, the crossproduct terms are negligible, and the two kinds
of equations are the same. But under neural conditions the crossproduct
terms are LARGE. For instance, effective inductance is more than 1012
times what we now assume it to be. The S-K equation predicts two modes
of behavior.
When G is high (some channels are open) behavior similar to that of
the current model is predicted.
When G is low, transmission has very low dissipation, and the system
is adapted to inductive coupling effects including resonance.
Under S-K, attenuation of waves per wavelength or per unit distance
varies over a much larger range than occurs in the now-accepted theory.
There is a low membrane conductance regime where attenuation of waves is
small, and wave effects are predicted. However, as channels open attenuation
increases enormously, and waves may be damped out in a few microns. Under
the new model a neural passage can be either sharply "on" or
sharply "off" depending on the degree of channel-controlled membrane
conductance. In the high g regime, attenuation per wavelength values
are qualitatively similar for the Kelvin-Rall and S-K.
Figures 2, and 3 plot unit wave amplitude after one wavelength (right
axis) or damping exponent per wavelength (left axis) as a function of membrane
conductance, g, for both Kelvin-Rall and S-K theory. The curves
map functions that move rapidly - exponents are graphed in log-log
coordinates. 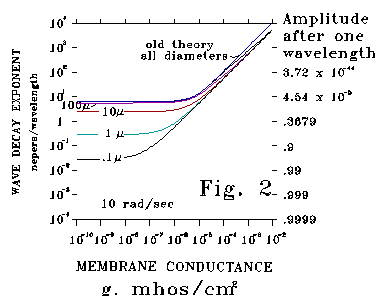
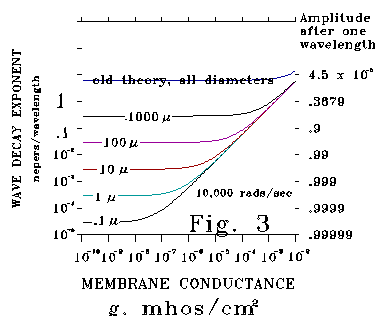
Figure 2 plots calculated responses at the low frequency of 10 radians/second
for neural process diameters ranging over five decades (from 1000 microns
to .1 micron). For the 1000 and 100 micron cases the Kelvin-Rall and
S-K curves are almost the same. Results for these large diameters and low
frequencies are also nearly the same on an attenuation per unit length
and a phase distortion basis. These conditions correspond to squid axon
experiments that are famous tests of the Kelvin-Rall theory. However,
for smaller diameters, attenuation according to S-K theory is much less
than that according to Kelvin-Rall.
Figure 3 plots calculated responses at 10,000 radians/second (1591 Hz)
for the same diameters plotted in Figure 2. Attenuation values are substantially
less for the new theory than for the Kelvin-Rall theory even for the 1000
micron diameter case. For Kelvin-Rall, the value of the attenuation exponent
per wavelength never falls below 2. This means that the maximum
amplitude of a wave after 1 wavelength of propagation is .00187 (about
1/535th) of its initial value under Kelvin-Rall for any diameter neural
process. It makes little sense to talk of "wave propagation"
and no sense to talk about "resonant responses" under these conditions.
In contrast, according to the new theory, as much as 99.995% of unit wave
amplitude may remain after a single wavelength. Under these very different
conditions, notions of wave propagation and resonance do make sense.
Fig. 5 plots conduction velocity versus frequency for a 1 micron dendrite or spine neck versus frequency in the case where membrane conductance, g, is approximately zero.
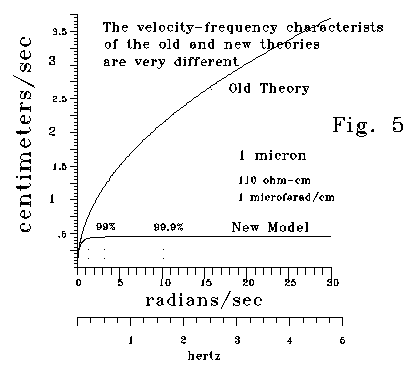
In the Kelvin-Rall theory, conduction velocity is proportional to the
square root of frequency. In the S-K model, conduction velocity rapidly
approaches an asymptote. Above a frequency threshold, conduction speed
is almost constant. For large diameter neural processes, this threshold
is so high that the velocity-frequency relation is similar for both theories.
But for small neural processes, velocity is almost constant above quite
low threshold frequencies. The following chart is based on a of 110 ohm-cm
and a membrane capacitance of 1 microfarad/cm2. For a .1 micron
dendrite, 99.99% of peak velocity occurs at a very low frequency (.0511
cycles/second.)
diameter Frequencies
for the following fractions of
(microns) Peak
velocity (radians/second)
95%
99% 99.99%
1000 1,320
3,160 32,120
100 132
316 3,212
10 13.2
31.6 321.2
1 1.32
3.16 32.12
.1 .132
.316
3.212
"The 99% velocity cutoff frequency according to the new model offers
a good basis for comparing phase distortion predictions between Kelvin-Rall
and the new theory. Phase distortion occurs when different frequency components
of a signal move at different speeds. Phase distortion can rapidly degrade
the information content of a signal. In Kelvin-Rall, phase distortions
are enormous. However, for the new model, in the low G limit, a
dendrite or axon will have a characteristic frequency 99 that
has 99% of maximum propagation velocity. Above 99, propagation
will be almost free of phase distortion. 99 correlates with
diameter, conductivity, and membrane capacitance according to the relation
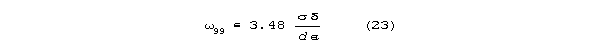 .
.
The S-K theory has new, interesting characteristics in its low g,
low attenuation mode. This may be described as an "on" state,
in contrast to the high g, high attenuation "off" state.
In the "on" condition, with g very small, important relationships
are simple, particularly for small neural diameters.
Components of important formulae are the "radical value" analogous
to the radius in the argand diagram characterizing the transmission line,
and the angle "" on that argand diagram. The radical for the
low g limit model is
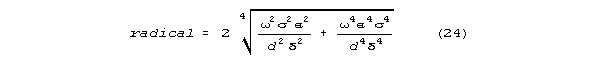
a neurally interesting characteristic emerges when this is factored
as follows:
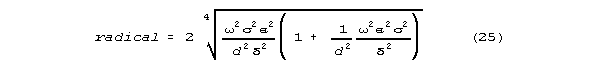
There is a term proportional to frequency (the fourth root of frequency4
) The relative importance of this term grows rapidly as diameter, d, decreases.
For dendrites and dendritic spines in brain, it is this frequency term
that predominates. The radical can be approximated as
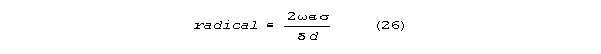
The rotational angle for the new theory is approximately
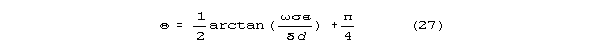
The quantity inside the arctangent above is strongly dependent on diameter
scale, resistivity, and frequency.
Attenuation per unit distance is
![]()
In the range where resonance is of interest (above the 99% of maximum
velocity frequency) the following approximations are useful:
For the wavelength :
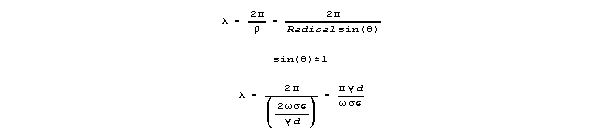
Attenuation per wavelength (in the max velocity range) is approximated
as follows:
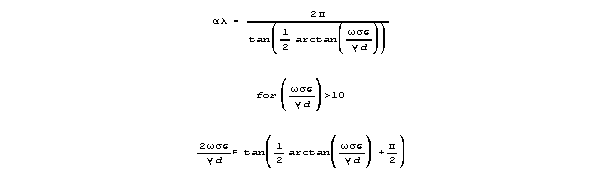
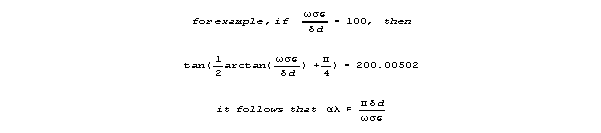
Velocity is
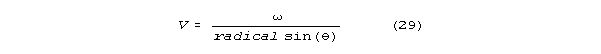
For frequencies that yeild near-maximum velocity, sin() is almost 1,
and

Impedance and Impedance Mismatch Effects:
Dendritic neural passages, which function as lossy transmission lines,
have impedance in the electrical engineering sense. Impedance may be defined
in the usual way, but with hatted values substituted for the unhatted ones.
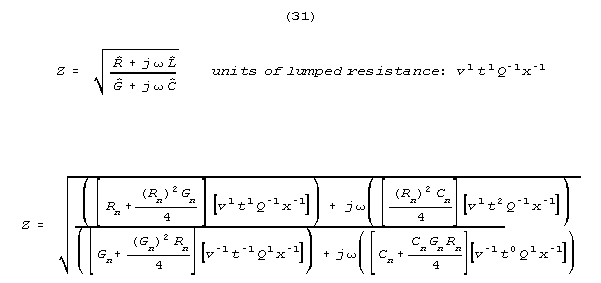
Note that impedance has the units of lumped resistance - (for the same
reason that coax and other wave carrying lines are specified in ohms, with
the ohmic values far in excess of the static resistances of the lines.)
The interface between two sections of transmission line generates reflections
unless their impedances are matched. For a line with an impedance Zo
terminated in another line (or lumped resistance) called the "load",
and having an impedance Zl, the reflection coefficient
Kl (the ratio of reflected to incident voltage at the load) will be
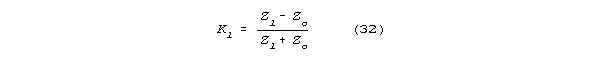
Reflection coefficient will vary from -1 to +1. When Zl>>>Zo
then Kl is approximately 1; when Zl<<<Zo
then Kl is approximately -1; when Zl
= Zo then Kl is 0, and there is no
reflection. Similar reflection rules and similar consequences are familiar
to microscopists and students of acoustics.
Impedance jumps in neural passages due to changes in crossection or
g are analogous to similar sharp impedance jumps that occur in wind
musical instruments. Wind instruments work because they are abruptly switchable
impedance mismatch devices driven by cyclic energy input means that adapt
a fluctuating input flow to match air column load. A sharply defined quasi-steady
resonance is established in the musical instrument's air column. Because
the reflection coefficient at the discontinuity is not perfect, some of
the sound from this column radiates to listeners of the music. Analogies
between the acoustical function of wind instruments and neural lines are
useful when considering two linked issues.
1) A sharp change in membrane conductance, g, (whether "inhibitory"
or "excitatory") can produce reflection as well as attenuation.
If the change is relatively large, this reflection can be both sharp and
strong.
2. Because reflecting discontinuities can be set up by synaptic or dendritic
spine activity, the neural structure can operate as an abruptly switchable
impedance mismatch device with variable resonance properties. This opens
possibilities for logical function.
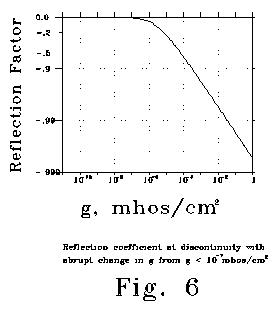
Consider a dendrite of 2 microns diameter, with a group of channels
of one hundred 200 picosiemen channels over a 1 micron axial distance.
Conditions are sigma=110 ohm-cm, capacitance/area=10-6 farads/cm2
,gclosed channel=10-12 mhos/cm2. When the
channels are closed, they do not effect conduction. Suppose that the channels
open so that g goes from 10-12 to some much higher value.
See Fig 6, which plots a change for reflection coefficient from 1.0 to
less than .0025. Fig 6 was calculated for 10 radians/second, but
values for much higher or substantially lower frequencies would be about
the same. Note that the difference in reflection coefficient that occurs
between 10-12 and 10-6 mhos/cm2 is small,
but that very large changes in reflection coefficient are calculated for
higher conductances. Opening a large number of channels in the side of
a dendritic passage can change the channel from a transmission line to
a sharply reflecting discontinuity. The physics is analogous to that which
occurs when a clarinettist opens or covers a finger hole.
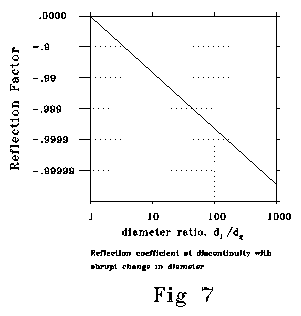
Abrupt changes in crossection are also calculated to produce analogous
reflecting impedance mismatches.
Fig 7 shows reflection coefficient at a discontinuity between a smaller
and a larger diameter. Calculated conditions are sigma= 50 ohm-cm, 10-6
farads/cm2, g=10-9, frequency=900 radians/sec,
d1=1 micron. The shape and slope of this function is
insensitive to changes in frequency, initial diameter, sigma, g
and c. Abrupt changes in crossection can produce strongly reflecting
impedance mismatches. When changes in crossection or impedance are gradual,
they can occur with little or no reflection, for reasons exactly analogous
to those that permit smooth transitions in the bells of wind instruments
or the gradual impedance (refraction) transitions that can be arranged
in optics and in waveguide practice. The S-K equations are switching, resonance
adapted equations well adapted to the information processing that brains
do.
NOTES AND REFERENCES (for the annotated Koch article and Appendices):
1. Regan, D. HUMAN BRAIN ELECTROPHYSIOLOGY: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine Elsevior, New York, Amsterdam, London 1989 pp 100-115.
2. Sejnowski, T.J. "The year of the dendrite" SCIENCE, v.275, 10 January 1997 pp 178
4. Zecevic, D. "Multiple spike-initiation zones in single neurons revealed by voltage-sensitive dyes NATURE v.381 23 May 1996
pp 322-324.
5. Regan, op. cit. Fig 1.70A and Fig 1.70B, pp 106-107.
6. The International Dictionary of Applied Mathematics D.Van Nostrand Company, Princeton, Toronto, New York, London 1960
8. C.H. Horner Plasticity of the Dendritic Spine, Progress in Neurobiology, v.41, 1993 pp. 281-231, Fig 3, p 285.
9. I believe that the extensive destruction of spines and dendrites that occurs in severe epilepsy may happen in this way.
10. I.B.Levitan and L.K.Kaczmarek THE NEURON: Cell and Molecular Biology Oxford University Press, Oxford, New York, 1991, p. 65-66.
11. When the derivation of the conduction equation from a finite "physical" model is carefully done, a series of crossterms arise in addition to the terms in R, L, G, and C. This has long been known, but the crossterms have been thought to be infinitesimal. However, when the rule that crossproducts in dimensional parameters must be evaluated in intensive (point) form is accounted for, these crossterms are finite. Crossterms that are large enough to effect neural conduction (which happen to go as the inverse cube of diameter) are included in the equation. The new crossterm parameters can be combined with the old term parameters in a hat notation. For instance, the hatted value of R includes R and a crossterm that, like R, is associated with i. The hatted value of L includes L and a crossterm that, like L, is associated with di/dt.